EU Regulation 1223/2009 is the main regulatory framework for finished cosmetics in the EU market, which not only affects retailers, importers, but also producers and raw material suppliers of products.
This article focuses on the concerns of our clients and interprets the most important content in the regulation.
Overview
Regulation requires product owners to prepare a safety report (PIF) before placing it on the market and uploading it to CPNP for notification, monitoring, and analysis. In addition, animal testing is not allowed on the product itself or its ingredients.
The regulation consists of 10 chapters, 40 articles, and 10 annexes:
| Chapter | Title | Articles |
| I | scope definitions | 1-2 |
| II | safety, responsibility, free movement | 3-9 |
| III | safety assessment, product information file, notification | 10-13 |
| IV | restrictions for certain substances | 14-17 |
| V | animal testing | 18 |
| VI | consumer information | 19-21 |
| VII | market surveillance | 22-24 |
| VIII | non-compliance, safeguard clause | 25-28 |
| IX | administrative cooperation | 29-30 |
| X | implementing measures, final provisions | 31-40 |
| annex | I-X |
CPNP
Cosmetic Products Notification Portal, an online notification system created for the EC1223, requires all products sold in the European Union to be registered with CPNP before being put on the market, and it is mandatory. Regulatory authorities of the European Union and member states can conduct market supervision, analysis, etc. on the information in CPNP.
Here is the information needed when submitting to CPNP:
- Product name and type
- Product owner’s name and address
- Country of Origin(if the product is imported)
- Picture of product outer packaging
- Product label photos
- Nanomaterial information (if available)
Application duration: 1-2 weeks, it can be seen that the information required for CPNP is actually PIF.
PIF
Product information file, it is a set of various documents and tests related to finished products, raw materials, packaging specifications, manufacturing processes, etc. Article 11 of EC1223 explains its content. Contains the following elements:
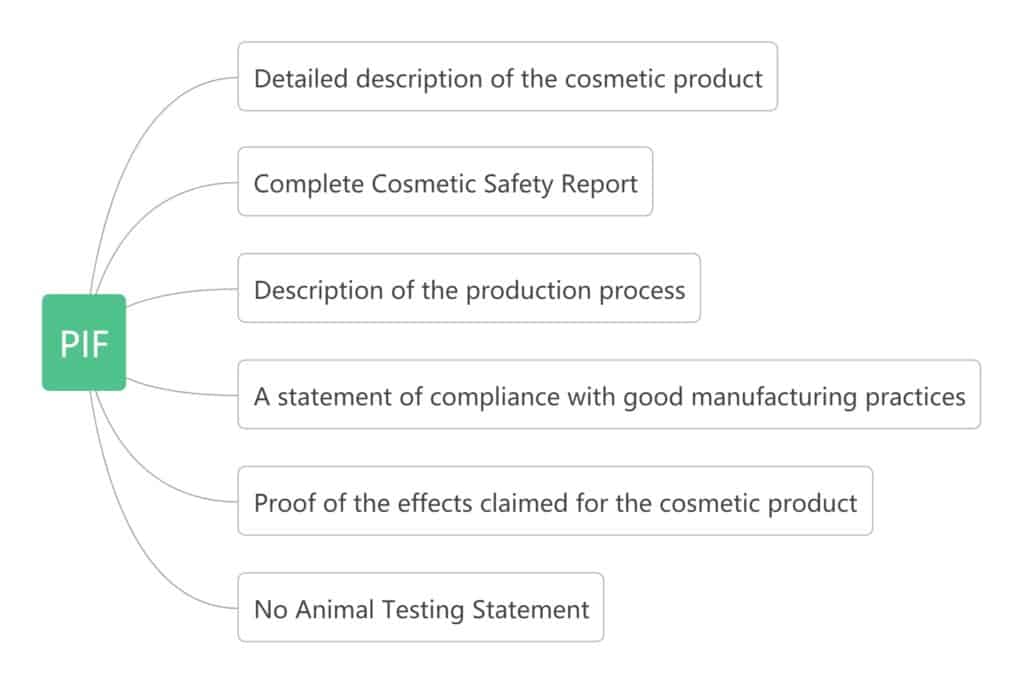
The person in charge is responsible for creating, storing, and maintaining this mandatory document, which must be kept in the file for 10 years after the last batch of products is launched on the market.
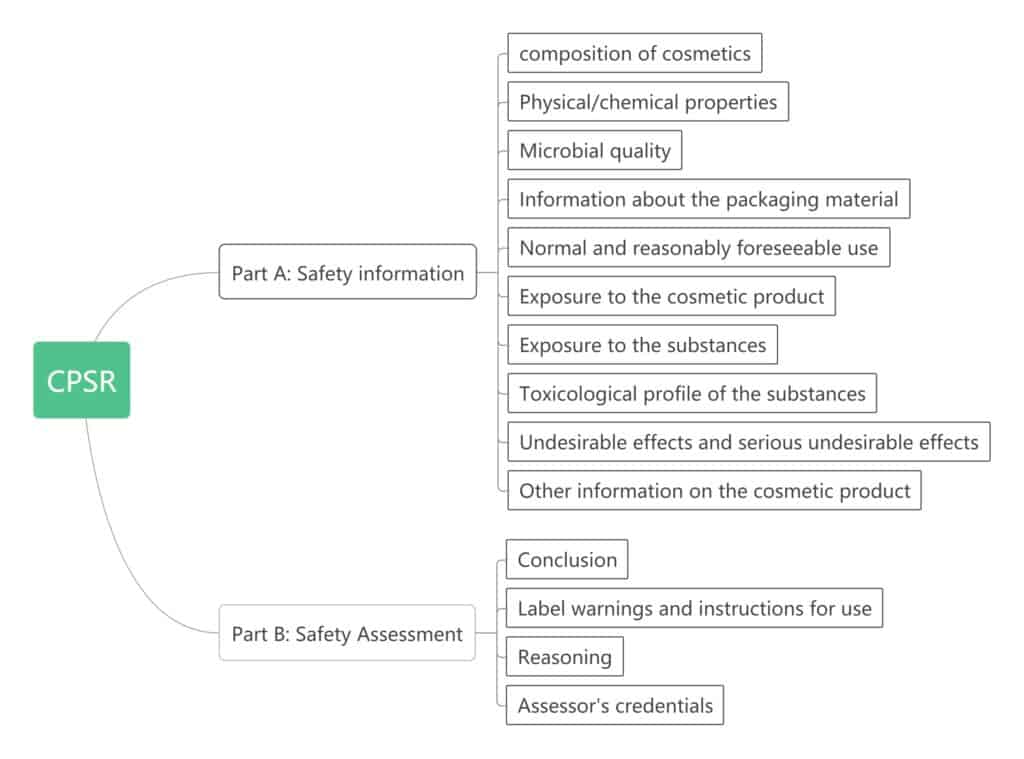
CPSR
Cosmetic product safety report, it is included in the PIF, the first part is provided by the manufacturer or responsible person, the second part writes according to the first part of the content and needs to be evaluated by a qualified evaluator. This part is described in detail in Appendix I of EC1223. learn more
Label
The regulations emphasize the importance of product packaging labeling. Consumers have the right to obtain various necessary product information, including product ingredients, through the label. There are detailed explanations in Chapter 6 of EC1223, to ensure that the label is indicated with the following content in a durable, clear, and eye-catching manner.
- The name and address or registered office of the responsible person, indicating the country of origin for products produced outside the European Union.
- The net content of the contents inside the container, expressed in weight or capacity. Content below 5g or 5ml can not be labeled.
- The expiration date of the product should be marked in the order of month and year. Cosmetics with a validity period of more than 30 months may not be labeled with an expiration date, but they should indicate the date of use when they are opened.
- Precautions, conditions, and warnings for use. The specifications for the symbols on the labels can be found in Appendix VIII.
- Product batch number or document number identifying the product.
- Product efficacy: If the appearance of the product can clearly display its efficacy, it may not be labeled.
- Ingredients information shall be named and labeled according to the cosmetics ingredients published by the European Union.
Ingredient
Ingredient is of utmost importance, and it can be said that the entire regulation revolves around the safety of ingredients to ensure the safety of the final product. In our daily projects, the safety review of ingredients is also the most time we spend.
The annex of the regulation lists a large amount of ingredient information as follows, which you can search through the EU Cosmetic Ingredients Database
- Annex II – list of banned substances
- Annex III – list of restricted substances
- Annex IV – list of allowed colorants
- Annex V – list of allowed preservatives
- Annex VI – list of allowed UV-filters



