Cosmetics production refers to the process in which factories convert raw materials, packaging materials, and other resources into cosmetics products that consumers can directly use through certain technological processes. A good production quality management system is the basic guarantee for producing safe and high-quality cosmetics, and GMPC is the guide of the quality management system.
Introduction of GMPC
GMPC is the abbreviation of “good manufacturing practice of cosmetic products”. Due to the significant effect of GMP in regulating the production of drugs/food, improving the quality of drugs/food, and ensuring the safety of drugs/food, the US FDA issued Cosmetic Good Manufacturing Practice Guidelines in 1992 to guide cosmetics production enterprises to regulate their production, thereby ensuring the hygiene and safety of cosmetics.
In July 1994, the European Union Federation of Cosmetics, Toiletries and Essence Industries (COLIPA) published the GMP guidelines for cosmetics.
In 1988, Japan issued the GMP Guidelines for Cosmetics as a self-discipline rule in the industry (Drug Administration No. 57 of August, Showa 63).
In 2003, Southeast Asian countries released the GMP for cosmetics.
In 2005, the European Federation of Cosmetic Ingredients (EFfCI) formulated the GMP for cosmetic ingredients.
Nowadays, cosmetics sold in the US and EU markets must comply with US federal cosmetics regulations or EU cosmetics directives to ensure the health of consumers after normal use.
In order to eliminate regulatory barriers to cosmetics in various countries or regions and ensure that cosmetics production maintains the highest production level to protect global consumers, the International Organization for Standardization (ISO) released ISO22716:2007 Cosmetics Food Manufacturing Practices (GMP).
Benefits of GMPC
The role benefits cosmetic companies in promoting GMPC include:
- Ensure product safety;
- Improve product quality;
- Eliminate dangerous accidents;
- Reduce the risk of product harm to consumers;
- Reduce the risk of public recycling of products;
- Compliance with regulations and trade guidelines;
- Good working environment;
- Effective cost control and international recognition;
- Enhance product competitiveness;
- Effective product traceability.
Content of GMPC
GMPC is a specific regulation made for the software and hardware aspects of the factory equipment, environment, personnel, and hygiene management/control of cosmetics manufacturers based on product characteristics. Although there are some differences in the entries and arrangements of Good Manufacturing Practice (GMPC) for cosmetics production in various countries, the main core components are similar.
GMPC in the United States
The main content of GMPC in the United States is as below figure. The 10 items listed the inspection content for each item. How to meet the requirements of the inspection content is determined by each company’s own appropriate method, which is relatively flexible. The regulations for records and documents only list the required documents and do not specify them. Cosmetics companies adopt different management systems that comply with GMP guidelines.
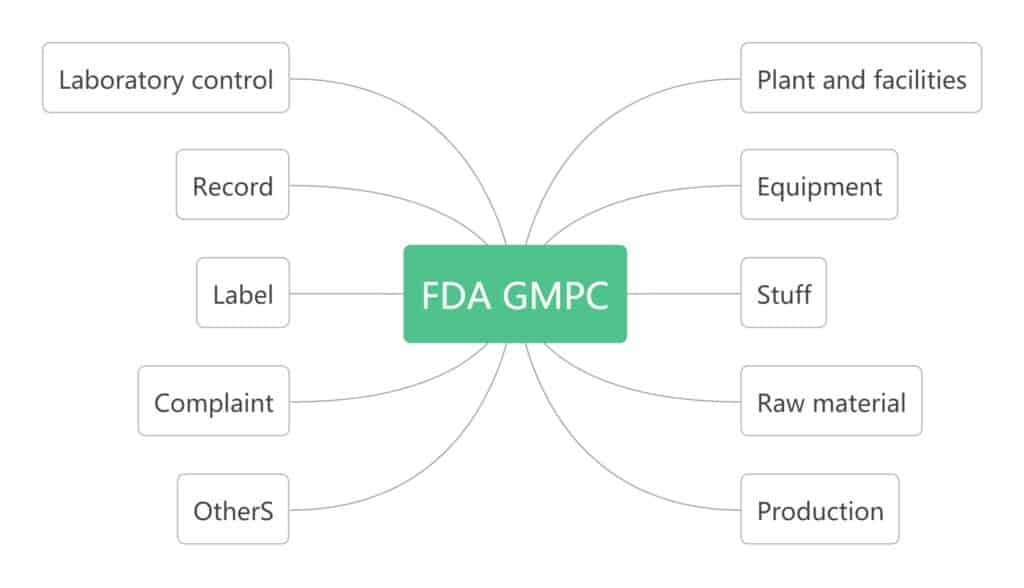
GMPC in European Union
EU GMPC believes that the most important thing is to predict quality defects, therefore it considers all elements within each manufacturing company together so that cosmetics can be produced efficiently, and conform to its own predetermined planning criteria. Cosmetic manufacturers can establish a comprehensive quality management system in accordance with GMP guidelines.
The EU GMPC is a measure that should be implemented after the product has been clearly identified and deliberated through the development stage and is limited to production, including:
- encouraging companies to propose a method to give their quality assurance a legal status;
- stipulating that the production process is different Some conditions that the stage should perform;
- describe some activities that lead to quality assurance.
The EU GMPC believes that GMP is the result of all professional experience, its purpose is its general significance, and it should not hinder improvement at any time. These improvements include:
- the development of related machinery, processing technology, packaging and control equipment;
- the development of manufacturing technology and packaging technology;
- the evolution of production organization.
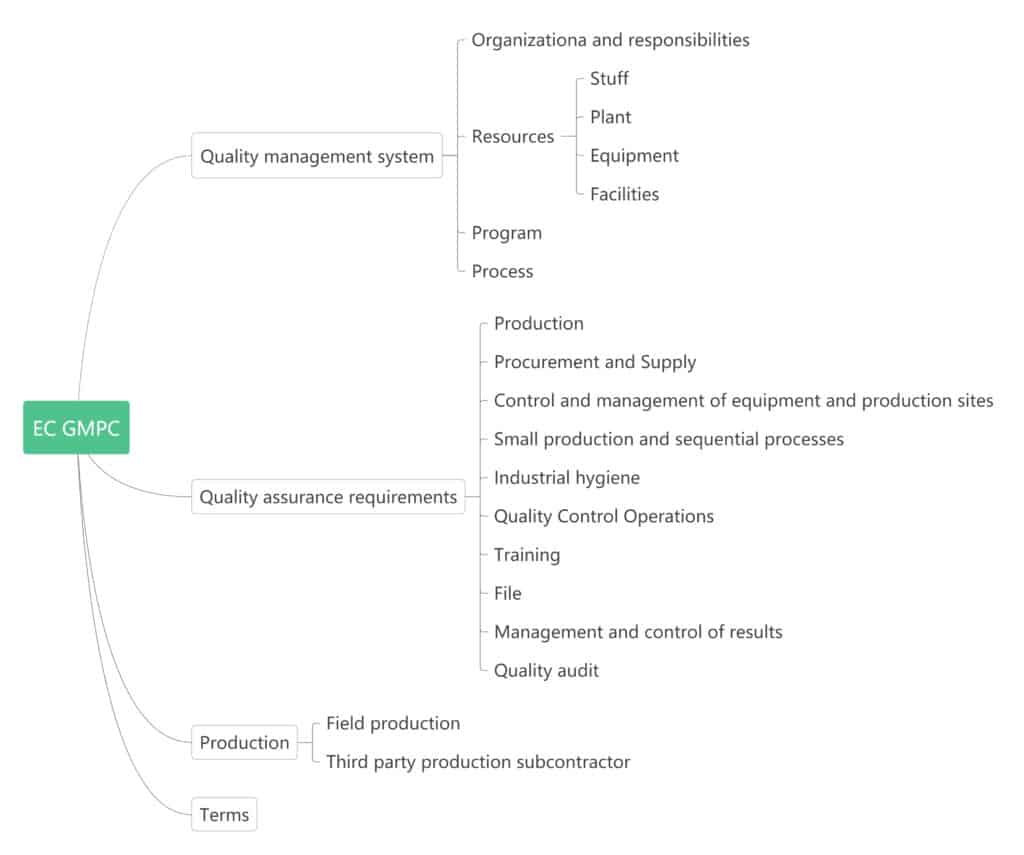
GMPC in ASEAN
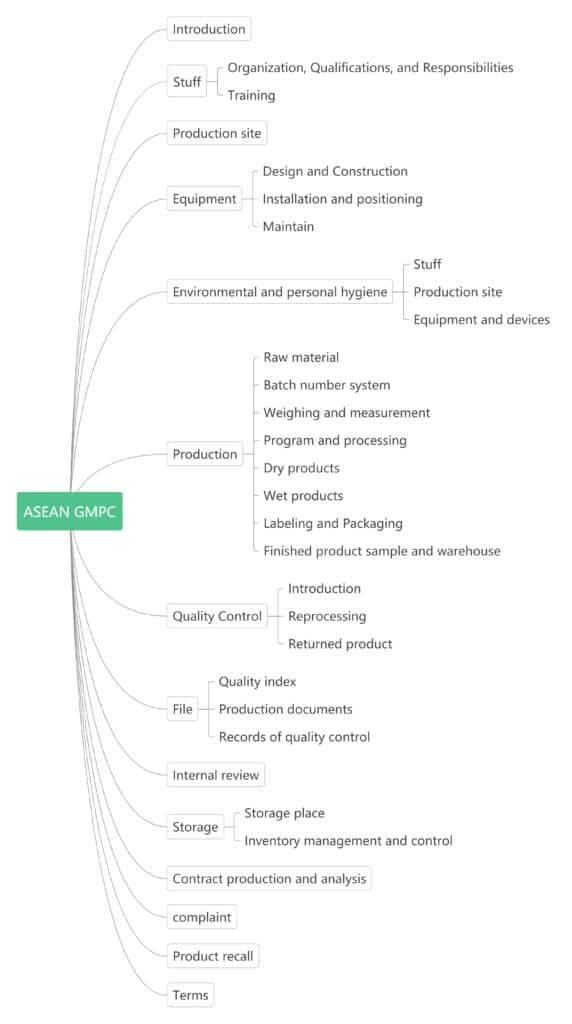
The ASEAN GMPC is similar to the EU GMPC, with more specific entries. Its purpose is to provide assistance to cosmetics companies that comply with Southeast Asian cosmetics regulations. It is just a general guide for manufacturers to develop their own internal management systems and procedures. The important purpose is to meet the quality standards corresponding to its intended use in all cases, ensuring the health and interests of consumers, and ensuring that the product is produced and controlled to a specific quality in a coordinated manner. It is related to all aspects of production and quality control.
ISO 22716:2007 GMPC
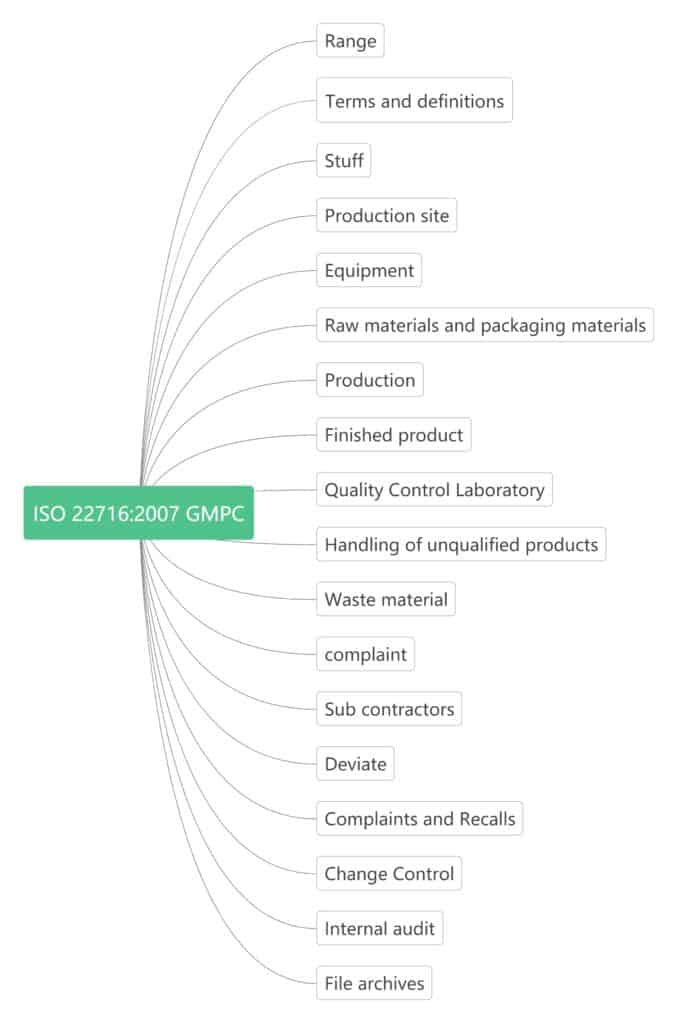
It is an International Standard drafted according to the rules provided by the ISO/IEC Directives, designed for the cosmetics industry and takes into account the special needs of the sector. These guidelines provide organizational and practical advice on people management, technology, and management factors affecting product quality.
ISO 22716:2007 GMPC proposes guidelines for production, control, storage and transportation. These guidelines cover product quality issues, but not the safety of workers employed in factories, nor environmental protection issues. Safety and environmental aspects are inherent corporate responsibilities and can be governed by local regulations and laws. These guidelines are not intended for research and development activities and product sales.
How to get GMPC certification?
To obtain GMPC (Good Manufacturing Practice for Cosmetics) certification, cosmetic manufacturers must follow a set of guidelines and procedures. Here are the basic steps you need to take to get GMPC certification:
- Conduct a self-assessment: Before seeking certification, it’s important to assess your current manufacturing practices and identify any areas where you may need to improve. You can use the GMPC guidelines as a reference to conduct your self-assessment.
- Develop a quality management system: You need to develop a quality management system that meets the GMPC guidelines. This system should cover all aspects of the manufacturing process, including raw material procurement, production, testing, packaging, and storage.
- Implement the quality management system: Once you have developed your quality management system, you need to implement it and ensure that all staff are trained and aware of the new procedures. This may involve making changes to your manufacturing process, including introducing new equipment, updating procedures, and training staff on new processes.
- Conduct internal audits: You need to conduct regular internal audits to ensure that your quality management system is working effectively and meeting the GMPC guidelines. This involves reviewing your manufacturing processes, documentation, and procedures to identify any areas where you may need to improve.
- Get certified: After implementing and refining your quality management system, you can seek certification from a third-party certification body that is accredited to certify against GMPC. The certification body will conduct an audit to verify that your quality management system meets the GMPC guidelines. If your system meets the required standards, you will be issued with a GMPC certificate.
It’s important to note that the specific requirements for obtaining GMPC certification may vary depending on the certification body and the country or region in which you operate.



